- Stage of development
-
Technology validated in the laboratory
- Intellectual property
-
Priority patent filed
- Intended collaboration
-
Licensing and/or co-development
- Contact
-
Alfonso del ReyVice-presidency for Innovation and Transferadelrey@icmab.escomercializacion@csic.es
- Reference
-
CSIC/AF/024
Additional information
#Health
#Medical device
#Therapy
#Materials
#Micro & Nanomaterial
#Health
#Oncology
Hydrogel for its use in CAR-T Therapy
Novel Hydrogel with optimized mechanical and structural properties to resemble artificial lymph nodes and improve primary human CAR-T cell manufacture.
- Market need
-
Adoptive cell therapy (ACT) is a novel immunotherapy against cancer. Chimeric antigen receptor CAR-T therapy is a promising ACT. T cells are harvested from the patients and are genetically modified to express an artificial receptor, the CAR, that selectively targets cancer cells. T cells are expanded ex vivo and re-infused in the patient to destroy cancer cells.
Gold standard ex vivo expansion methods cannot mimic the proliferation and activation of T cells that occurs in vivo in the lymph nodes. A major challenge in CAR-T therapy is to manufacture large amounts of persistent therapeutic T cells.
Los métodos de cultivo de referencia no pueden imitar la proliferación y activación de las células T que ocurre in vivo en los nódulos linfáticos. Un gran reto de la terapia CAR-T es la producción de grandes cantidades de células T terapéuticas.
- Proposed solution
-
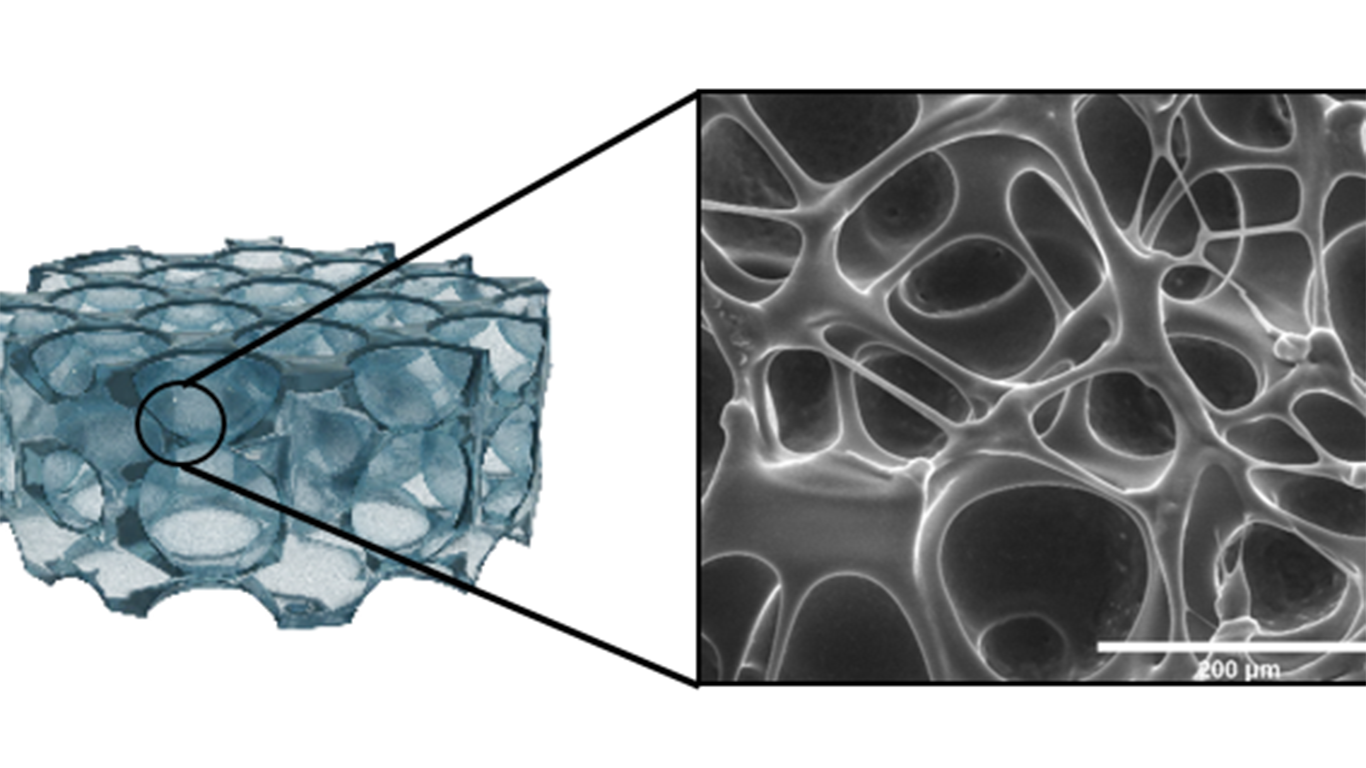
This novel hydrogel has mechanical and structural properties that mimic the lymph nodes, which enhanced CAR expression and T cell proliferation. That is crucial, because CAR T ACT needs a sufficient expansion of cells, but also to ensure that as many cells as possible are expressing the CAR receptor.
- Competitive advantages
-
- Production of CAR T products with a higher number of cells expressing CAR receptors.
- Reduction of the time needed to obtain sufficient cells for the treatment.
- Easy-to-produce and easy-to-handle material.