- Stage of development
-
Pre-clinical in vivo
- Intellectual property
-
PCT application
- Intended collaboration
-
Licensing and/or co-development
- Contact
-
Eva GabaldónVice-presidency for Innovation and Transfereva.gabaldon@csic.escomercializacion@csic.es
- Reference
-
CSIC/EG/127
Additional information
#Health
#Therapy
#Antibodies
#Immunology
Antibody-Drug Conjugates and CAR-T cells for the selective treatment of T-ALL
A new immunotherapy strategy based on the administration of ADCs or CAR-T cells derived from a monoclonal antibody specific for the pre-TCR receptor, which impairs LIC activity and tumor progression, has been developed and validated in a preclinical human T-ALL xenotransplantation model in mice.
- Market need
-
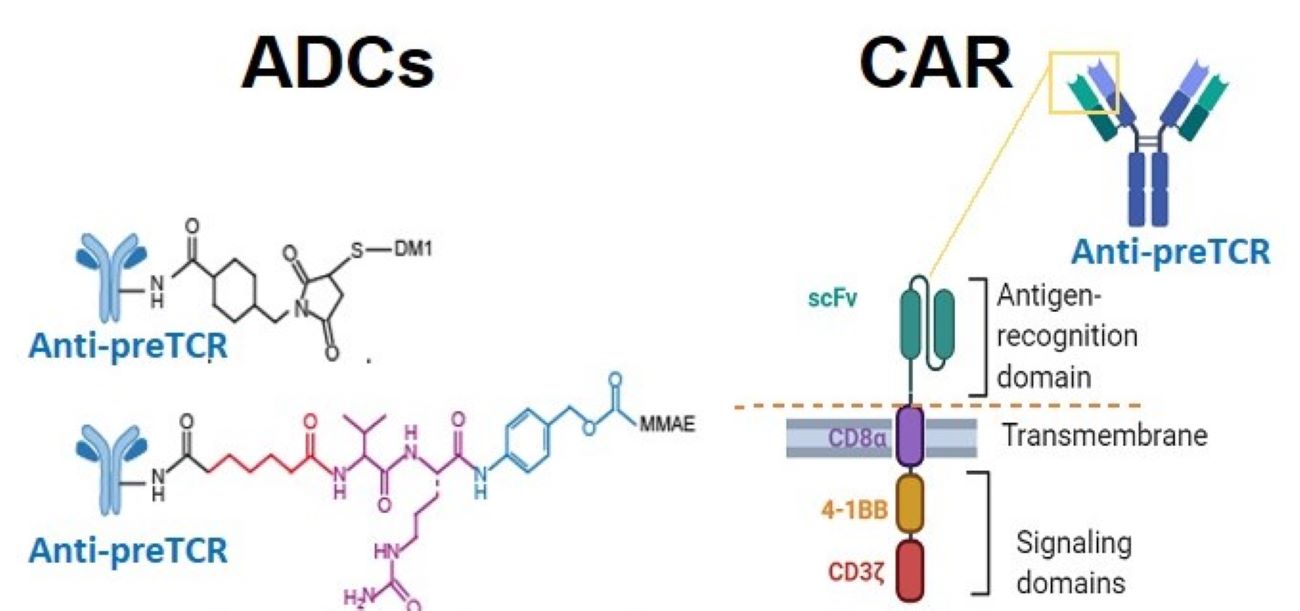
Targeted immunotherapies based on monoclonal antibodies (mAbs) or T cells armed with chimeric antigen receptors (CAR-T) remain challenging for T-cell acute lymphoblastic leukemia (T-ALL), because of the lack of specific therapeutic targets that are selectively expressed on leukemic T cells, but not on normal T cells. The present technology relates to an antibody, or an antigen binding fragment thereof, and to the said antibody bound to a cytotoxic agent (antibody-drug conjugate, ADC), or to a CAR T-cell comprising the antigen binding fragment of said antibody, which binds the pTα subunit of the human pre-T cell receptor (pre-TCR), a surface receptor expressed in developing thymocytes and >50% T-ALL cases, but not in normal T cells.
- Proposed solution
-
This therapeutic target provides growth advantage to malignant T-ALL cells and displays dynamic endocytic properties. The specific ADCs and CAR-Ts are useful for targeting and killing T-ALL cells that express pre-TCR and display Leukemia Initiating Cell (LIC) activity. Moreover, administration of ADCs or CAR-Ts is validated as a selective targeted immunotherapy for human pre-TCR+ T-ALL, as it effectively impairs LIC activity and tumor progression and improves survival of treated mice in preclinical in vivo models.
- Competitive advantages
-
- The proposed targeted immunotherapy overcomes T-cell aplasia and CAR-T fratricide.
- The immunotherapy targets a receptor expressed in T-ALL leukemia initiating cells, and is thus optimal for treatment of relapsed T-ALL.
- The targeted receptor is optimal for an ADC therapeutic strategy, owing to its dynamic internalization properties.
- The therapeutic target contributes to T-ALL cell survival and proliferation, and thus emergence of target-negative escape mutants will be unlikely.