- Stage of development
-
Validation with patients’ samples of different viral infections is ongoing. Developing prototype.
- Intellectual property
-
Patent application filed in USA and Europe, not yet granted
- Intended collaboration
-
Licensing and/or co-development
- Contact
-
Isabel MasipVice-presidency for Innovation and Transferisabel.masip@csic.escomercializacion@csic.es
- Reference
-
CSIC/IM/075
Additional information
#Health
#Diagnostics
#Biomarker
#Respiratory disease
#Infectious disease
PCR-less technologies for rapid detection of respiratory viruses
Novel DNA probes useful for the rapid detection of SARS-CoV2, influenza and respiratory syncytial viral RNAs. Integrated in different set of devices, these probes enable the detection of RNA sequences in few minutes, without the need for RNA extraction, purification and PCR amplification
- Market need
-
(Rapid and efficient testing is required to effectively control SARS-CoV-2, influenza A virus (H1N1) or respiratory syncytial virus (RSV). RT-qPCR is the current gold standard for population-scale testing. Although highly specific, its sensitivity in clinical practice is only 70% and requires long turnaround times (TAT) because of the need to extract the sample and amplify the viral RNA. Tests are performed on centralized laboratories, thus delaying even more the delivery of the tests results. Other diagnostic technologies, such as antigen tests, can provide rapid results but present lower sensitivity and specificity.
- Proposed solution
-
A fast, sensitive and reliable biochemical approach is presented allowing to perform the test at the point of care, without specialized equipment or highly trained personnel. It also can be used on multiplexed high-throughput laboratory platforms to increase the efficiency and reduce TAT.
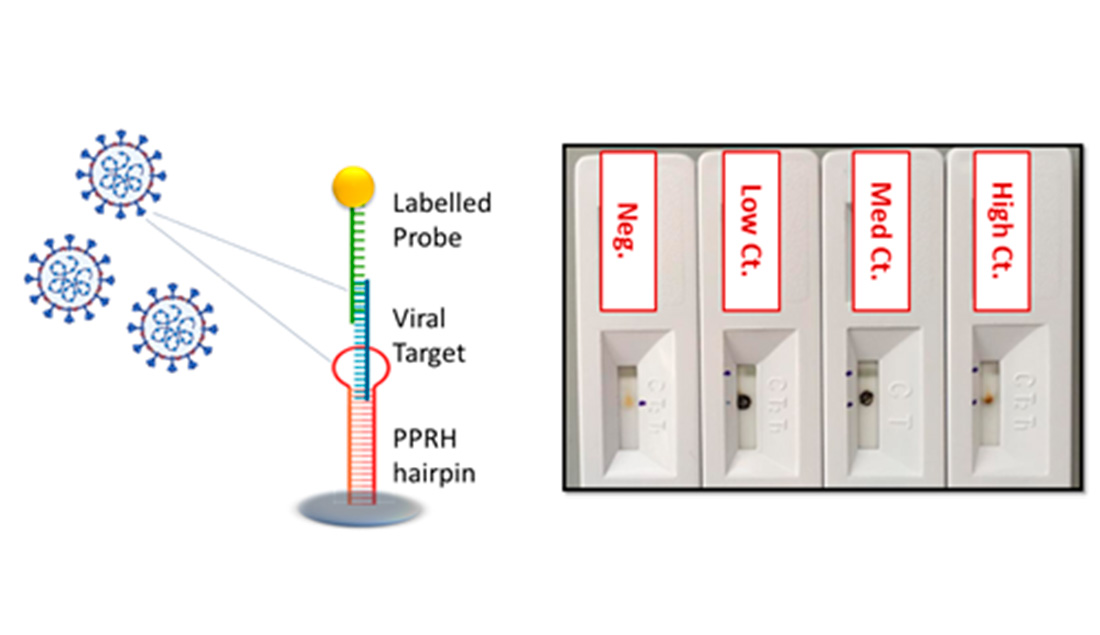
Innovative DNA probes, specific for each type of virus (RSV, H1N1 or SARS-CoV-2) have been designed, based on polypurine reverse Hoogsteen hairpins (PPRHs), The probes show high affinity for the viral RNA by forming triplex structures that present improved stability, allowing detection with high sensitivity without need for amplification.
- Competitive advantages
-
- RNA virus quantification can be achieved in less than 30 min following sample collection, without need for RNA extraction, purification and PCR amplification., sin requerir extracción, purificación o amplicación del RNA por PCR.
- High sensitivity, comparable to that determined by RT-PCR in clinical samples obtained from nasopharyngeal swabs of patients infected (up to 34 Ct, femtoM).
- Four different diagnostic devices have been optimized (thermal lateral flow PoC, electrochemical PoC biosensor, ELISA plate and fluorescent microarray).